Natural molecule could speed up battery recharging
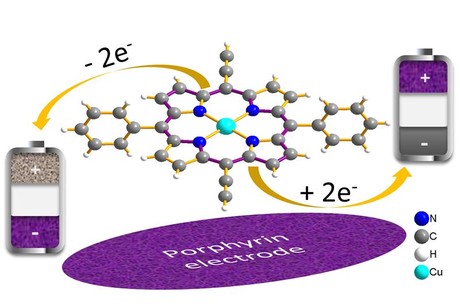
German researchers have found that the porphyrin molecule, on which chlorophyll, blood and vitamin B12 are based, can be used as an electrode material that speeds up the charging process of rechargeable batteries.
The most widely used battery technology in the world right now is based on lithium ions, as no other rechargeable storage device for electric energy has comparable properties. Thus, lithium-ion batteries are currently indispensable for devices such as laptops, smartphones and cameras, even though improved properties such as quick charging would be desirable.
Many materials that improve the properties of lithium-ion batteries do exist, but these are typically rare, expensive, toxic or harmful to the environment. Ideally, high-performance energy storage materials would be based on renewable resources.
Now, an interdisciplinary research group headed by Professor Maximilian Fichtner and Professor Mario Ruben, from the KIT Institute of Nanotechnology, presents a new energy storage material that enables the very fast and reversible inclusion of lithium ions. Their work has been published in the journal Angewandte Chemie International Edition.
“Porphyrins occur very often in nature and are the basic constituents of chlorophyll, of human and animal blood pigment (haemoglobin), and of vitamin B12,” Professor Fichtner said. Technical variants of these materials are already in use, eg, for blue-coloured toner in laser printers or for car paint. By bonding functional groups to porphyrin, the scientists managed to leverage its specific properties in electrochemical electric storage systems for the first time.
Functional groups were added to the organic copper porphyrin molecule, which produces structural and electroconductive cross-linking of the material when the battery cell is charged for the first time. This significantly stabilises the structure of the electrode in lab tests and allows several thousands of charge-discharge cycles.
With this material, storage capacities of 130–170 milliamp hours per gram (mAh/g) were measured in the lab — at a medium voltage of 3 V — along with charging-discharging times of only 1 min. Current experiments suggest that the storage capacity can be increased by another 100 mAh/g and that the storage system can be operated not only with lithium, but also with the much more abundant sodium.
“The storage properties are exceptional, because the material has the storage capacity of a battery but works as fast as a supercapacitor,” Professor Fichtner said.
Computational model enhances battery safety
Researchers have developed a new computational model that offers insights into one of the key...
Novel method to extend lifecycle of Li-ion batteries
Researchers have uncovered a hidden surface degradation mechanism in the cathodes of lithium-ion...
Sensor could help prevent Li-ion battery fires
Researchers have developed new technology to detect dangerous gas leaks in lithium-ion batteries,...





