More stable catalysts for hydrogen fuel cells
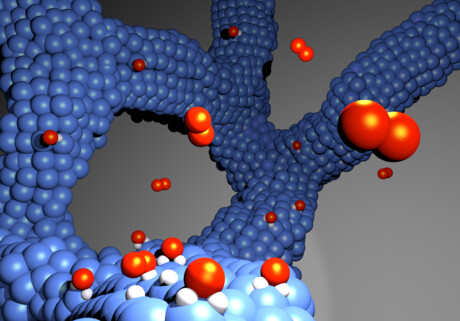
European researchers, led by the University of Bern’s Professor Matthias Arenz, have developed an electrocatalyst for hydrogen fuel cells that does not require a carbon carrier and is therefore much more stable than the catalysts commonly used today. Described in the journal Nature Materials, the new process is industrially applicable and can be used to further optimise fuel cell-powered vehicles without CO2 emissions.
In a hydrogen fuel cell, hydrogen atoms are split to generate electrical power directly from them. For this purpose, hydrogen is fed to an electrode, where it is split into positively charged protons and negatively charged electrons. The electrons flow off via the electrode and generate electric current outside the cell, which drives a vehicle engine, for example. The protons pass through a membrane that is only permeable to protons and react on the other side on a second electrode coated with a catalyst (here from a platinum–cobalt alloy network) with oxygen from the air, thus producing water vapour. This is discharged via the ‘exhaust’.
For the fuel cell to produce electricity, both electrodes must be coated with a catalyst. Without a catalyst, the chemical reactions would proceed very slowly. This applies in particular to the second electrode, the oxygen electrode. However, the platinum–cobalt nanoparticles of the catalyst can ‘melt together’ during operation in a vehicle. This reduces the surface of the catalyst and therefore the efficiency of the cell. In addition, the carbon normally used to fix the catalyst can corrode when used in road traffic. This affects the service life of the fuel cell and consequently the vehicle.
“Our motivation was therefore to produce an electrocatalyst without a carbon carrier that is nevertheless powerful,” Prof Arenz said. Similar catalysts without a carrier material have always had a reduced surface area — but since the size of the surface area is crucial for the catalyst’s activity and hence its performance, these were not considered suitable for industrial use.
The researchers were able to turn their idea into reality thanks to a special process called cathode sputtering, in which a material’s individual elements (here platinum or cobalt) are dissolved (atomised) by bombardment with ions. The released gaseous atoms then condense as an adhesive layer.
“With the special sputtering process and subsequent treatment, a very porous structure can be achieved, which gives the catalyst a high surface area and is self-supporting at the same time,” said lead author Dr Gustav Sievers, from the Leibniz Institute for Plasma Science and Technology. This electrocatalyst consists of a thin metal network and is therefore more durable: “A carbon carrier is therefore superfluous,” Dr Sievers said.
According to Prof Arenz, “The catalyst we have developed achieves high performance and promises stable fuel cell operation even at higher temperatures and high current density.” He added that the technology is “industrially scalable and can therefore also be used for larger production volumes, for example in the automotive industry”, allowing the hydrogen fuel cell to be further optimised for use in road traffic.
“Our findings are consequently of importance for the further development of sustainable energy use, especially in view of the current developments in the mobility sector for heavy goods vehicles,” Prof Arenz said.
Please follow us and share on Twitter and Facebook. You can also subscribe for FREE to our weekly newsletter and bimonthly magazine.
Machine learning enhances perovskite solar cell production
Researchers have demonstrated that machine learning can be used to improve the data analysis...
Nanoparticle breakthrough for longer-lasting perovskite solar
Researchers have developed a flexible solar cell that lasts nearly 10 times longer than others of...
Cracking the code on solid-state batteries
Using a combination of advanced imagery and ultra-thin coatings, researchers from the University...





