Scientists go with their gut on next-gen battery
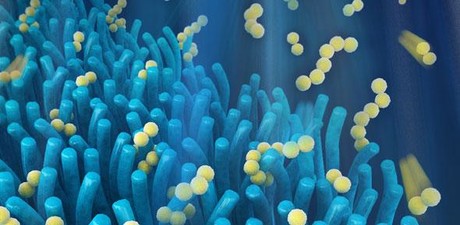
Scientists at the University of Cambridge have developed a prototype of a next-generation lithium-sulfur battery, inspired by the cells lining the human intestine.
To understand the need for a lithium-sulfur battery in the first place, we must start by taking a look inside a typical lithium-ion battery. It is made of three separate components: an anode (negative electrode, typically made of graphite), a cathode (positive electrode, typically made of lithium cobalt oxide) and an electrolyte. Positively charged lithium ions move back and forth from the cathode, through the electrolyte and into the anode.
The crystal structure of the electrode materials determines how much energy can be squeezed into the battery; for example, each carbon atom can take on six lithium ions, limiting the maximum capacity of the battery. Sulfur and lithium react differently, via a multi-electron transfer mechanism, meaning that elemental sulfur can offer a much higher theoretical capacity — and a lithium-sulfur battery would have much higher energy density.
However, when the battery discharges, the lithium and sulfur interact and the ring-like sulfur molecules transform into chain-like structures, known as a polysulfides. As the battery undergoes several charge-discharge cycles, bits of the polysulfide can go into the electrolyte, so that over time the battery gradually loses active material. It is this degradation that is currently hindering the commercialisation of lithium-sulfur batteries.
Working with the Beijing Institute of Technology, the Cambridge researchers developed a lightweight, nanostructured material which resembles villi, the finger-like protrusions which line the small intestine. In the human body, villi are used to absorb the products of digestion and increase the surface area over which this process can take place.
The prototype battery saw a layer of material with a villi-like structure, made from zinc oxide nanowires, placed on the surface of the cathode. This can trap fragments of the active material when they break off, keeping them electrochemically accessible and allowing the material to be used for longer. According to study co-author Dr Paul Coxon, “This gets us a long way through the bottleneck which is preventing the development of better batteries.”
The concept was trialled using commercially available nickel foam for support, which was later replaced by a lightweight carbon fibre mat to reduce the battery’s overall weight. Co-author Dr Yingjun Liu noted, “Changing from stiff nickel foam to flexible carbon fibre mat makes the layer mimic the way the small intestine works even further.”
Writing in the journal Advanced Functional Materials, the researchers stated that commercial variants of the battery would have five times the energy density of the lithium-ion batteries currently used in smartphones and other electronics. And while their version is still not able to go through as many charge cycles as a lithium-ion battery, the increase in energy density may cancel out the lower total number of charge-discharge cycles.
“This is the first time a chemically functional layer with a well-organised nano-architecture has been proposed to trap and re-use the dissolved active materials during battery charging and discharging,” said lead author Teng Zhao. “By taking our inspiration from the natural world, we were able to come up with a solution that we hope will accelerate the development of next-generation batteries.”
Soft robot uses magnetic fields to power itself autonomously
Inspired by the movement of manta rays, researchers have developed a small, magnetically powered...
Perovskite 'energy sandwich' could power next-gen solar
Researchers have achieved a new level of control over the atomic structure of halide perovskites,...
Creating the truck of tomorrow
Exploring the technological innovations, infrastructure solutions and emerging delivery methods...




