Sticking materials together without glue or tape — just electricity
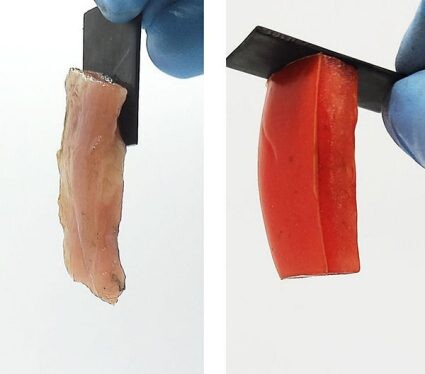
A study published in ACS Central Science has revealed that applying a small voltage to certain objects forms chemical bonds that link the objects together. Reversing the direction of electron flow easily separates the two materials. This electroadhesion effect could help create biohybrid robots, improve biomedical implants and enable new battery technologies.
When an adhesive is used to attach two things, it binds the surfaces through mechanical or electrostatic forces. But those attractions, or bonds, can be difficult to undo. As an alternative, reversible adhesion methods are being explored, including electroadhesion (EA), a process that involves running an electric current through two materials causing them to stick together due to their attractions or chemical bonds. Previously, Srinivasan Raghavan and colleagues demonstrated that EA can hold soft, oppositely charged materials together and can be used to build simple structures. Now, they have conducted tests to see if EA could reversibly bind a hard material, such as graphite, to a soft material, such as animal tissue.
The researchers tested EA using two graphite electrodes and an acrylamide gel. A small voltage (five volts) was applied for a few minutes, causing the gel to permanently adhere to the positively charged electrode. The resulting chemical bond was so strong that when the researchers tried to wrench the two pieces apart, the gel tore before it disconnected from the electrode. When the current’s direction was reversed, the graphite and gel easily separated and the gel instead adhered to the other electrode, which was now positively charged. Similar tests were run on a variety of materials, such as metals, animal tissues, fruits and vegetables, to determine the phenomenon’s ubiquity.
For electroadhesion to occur, the hard material needs to conduct electrons and the soft material needs to contain salt ions. The researchers hypothesise that the adhesion arises from chemical bonds that form between the surfaces after an exchange of electrons. This may explain why some metals that hold on to their electrons strongly, including titanium, and some fruits that contain more sugar than salts, failed to adhere in some instances. A final experiment revealed that EA can also occur underwater, thereby enabling a range of possible applications.
Preventing radiation-induced faults in electronics
Researchers are developing an open-source tool to enhance the prevention of radiation-induced...
QUT to establish photochemical mass spectrometer
A new mass spectrometry facility will be established in Queensland to enable real-time...
Enhancing computer cooling with ionic technology
Researchers have developed a new cooling technique for computer chips, using nanoscale channels...




