Researchers explore potential of MXenes for nanotech applications
A research team led by University of Nebraska–Lincoln materials scientists is exploring the physical properties of MXenes, a fast-growing family of two-dimensional materials with potential for many nanotechnology applications.
The team’s work builds on about two decades of research into graphenes, another family of 2D materials with important uses across many domains, but which exhibit some shortcomings compared to MXenes (pronounced “maxenes”).
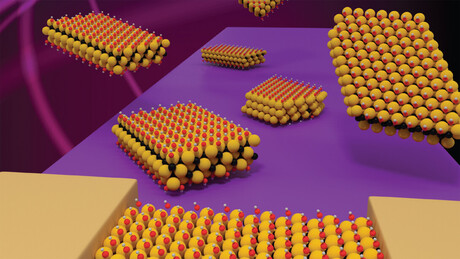
MXenes are made of atomically thin layers of transition metal carbides, nitrides or carbonitrides. They originate in what’s called the MAX phase, whose name describes its signature components: the “M”, a transition metal such as titanium or chromium; an “A” element such as aluminium; and the “X”, representing carbon or nitrogen atoms. The components are packed into a layered structure. To synthesise MXenes, chemists have used acidic solutions to etch away the layers of “A” elements while leaving the other layers intact — a relatively simple, high-yield technique.
Several dozen MXenes with different combinations of “M” and “X” elements have been synthesised. The Nebraska team has focused on one little-studied version that contains chromium, titanium and carbon atoms, said Alexander Sinitskii, professor of chemistry and lead researcher.
“The field is rapidly growing,” Sinitskii said.
MXenes have proved useful in storing energy, purifying water, protecting against electromagnetic interference, biomedical applications and more.
The keys to their usefulness are their chemical and structural diversity, as well as their scalability and processability, said Sinitskii, who also is affiliated with the Nebraska Center for Materials and Nanoscience, a nationally recognised centre of excellence that draws upon research expertise from three of the four University of Nebraska campuses.
MXenes have a large surface area and are easily tuneable. They also have a strong response to light and are hydrophilic — attracted to water — due to their oxygen- and hydroxyl-terminated surfaces.
Sinitskii’s team has discovered that the MXene containing chromium and titanium has “a certain set of properties not seen in others”. Previous research by the Nebraska team on other MXene materials revealed their n-type (electron-rich) character and decreased conductivity in response to light. In contrast, the new material is the first MXene with demonstrated p-type (electron-deficient) property and increasing conductivity under illumination.
“These are very unusual characteristics for MXenes,” Sinitskii said. “In many electronic applications, both n- and p-type materials are required and used in combination. Previously studied MXenes were all n-type, but now we demonstrate the first p-type MXene. This should enable complex structures where complementary MXenes are used together to achieve new electronic functionalities.”
His team also has been able to produce larger, more uniform flakes of the chromium/titanium carbide MXene than previously available, which makes them easier to research and use.
The team’s research was published in the journal Matter.
Quantum-inspired wireless tech to boost 6G performance
Researchers have developed a quantum-inspired method for optical wireless communication, to...
Internal quantum batteries to power scalable quantum CPUs
A new quantum-battery‑powered architecture, developed by researchers from CSIRO and the...
Porous gold metamaterial tunes electronic behaviour
By changing the physical structure of gold at the nanoscale, researchers can drastically change...




